Automated Liquid Chromatography

Best Practices and Innovations for Fully Automated Small Molecule LC
Available in presentation (PDF) file here for downloading and sharing.
- “Product Scale” Analysis & Purification
- “Intermediate Scale” Purification
- Final QC Analysis
Table Stakes for Automation
Human Tasks Are Asynchronous
- The system operates on-demand without human intervention
- Human intervention occurs at convenient times and does not disrupt on-demand system execution
Automation Friendly Software
- Sample / Sequence Data IN
- Start / Stop / Observability
- Sample Raw Data and Results OUT
Automation Compatible Hardware
- Samples IN & OUT
- Fractions OUT
Robustness” defaultOpen=>**
- Wide variety of unknown samples
- Low failure / intervention rate (< 0.2%)
- Resource Pooling - parallelism and fail-over protection
Opinions and Advice
The opinions and detailed system designs presented below are vendor agnostic - there is NO element of marketing, promotion, or relationship prioritization between Harman Solutions and ANY vendor including those in the space of analytical instrumentation.
That said, some specific products are identified in the materials below where there is ONLY ONE commercial option available with the required capabilities for the most robust and efficient fully automated chromatography systems. Where a product is specified, it is not opinion, but facts derived from extensive due diligence on all hardware and software systems available commercially.
Don’t make (and purify) material you don’t know you need
- Purification costs more and takes longer as scale increases (even with optimized systems), leaving you the ultimate choice to reduce quality or throughput
- The better option is to scale down synthesis - but using automated synthesis with well defined, reproducible synthetic routes and retaining intermediates, scale up can be done AFTER test results justify the effort
Analyze it before you purify it
- Knowledge gained from analyzing your reaction outcomes are necessary to improve automated synthetic methods
- Time spent on analysis will be gained in things NOT purified by implementing automated, data driven sample triage within your lab orchestration software
- Use analysis data to set injection volumes, collection trigger mechanism, collection thresholds, fraction volumes, peak counts, select methods
- Do the analysis and purification for the same sample on the same system whenever possible to reduce movements and system to system variability in hardware, mobile phases, communications
Don’t treat every sample as an exception
- Eliminate work-up and sample prep where possible (e.g. extraction) with robust chromatography system configurations
- Focus on identifying exceptions rather than treating everything as an exception (e.g. NMR analysis) after you have generated test data to justify the effort
“wasting resources more efficiently” - decreasing cost or improving capacity to produce unnecessary material will remain unnecessary
“Final Product Scale” Analysis and Purification
System design optimized for fully automated DMTA at scales of 3 - 100mg or 0.006 - 0.2mMol
System Design Objectives
- Analysis of crude reaction mixtures of any scale and concentration and at any time-points of chemical reactions (pre-reaction, mid-reaction samples, post-reaction, post-workup)
- Isolation (purification | extraction) of one or more specific molecules from a crude mixture with maximum yield and purity in minimum time
System Solution Design
Reverse-phase LC-MS with combined analysis and purification capabilities and sample quantitation
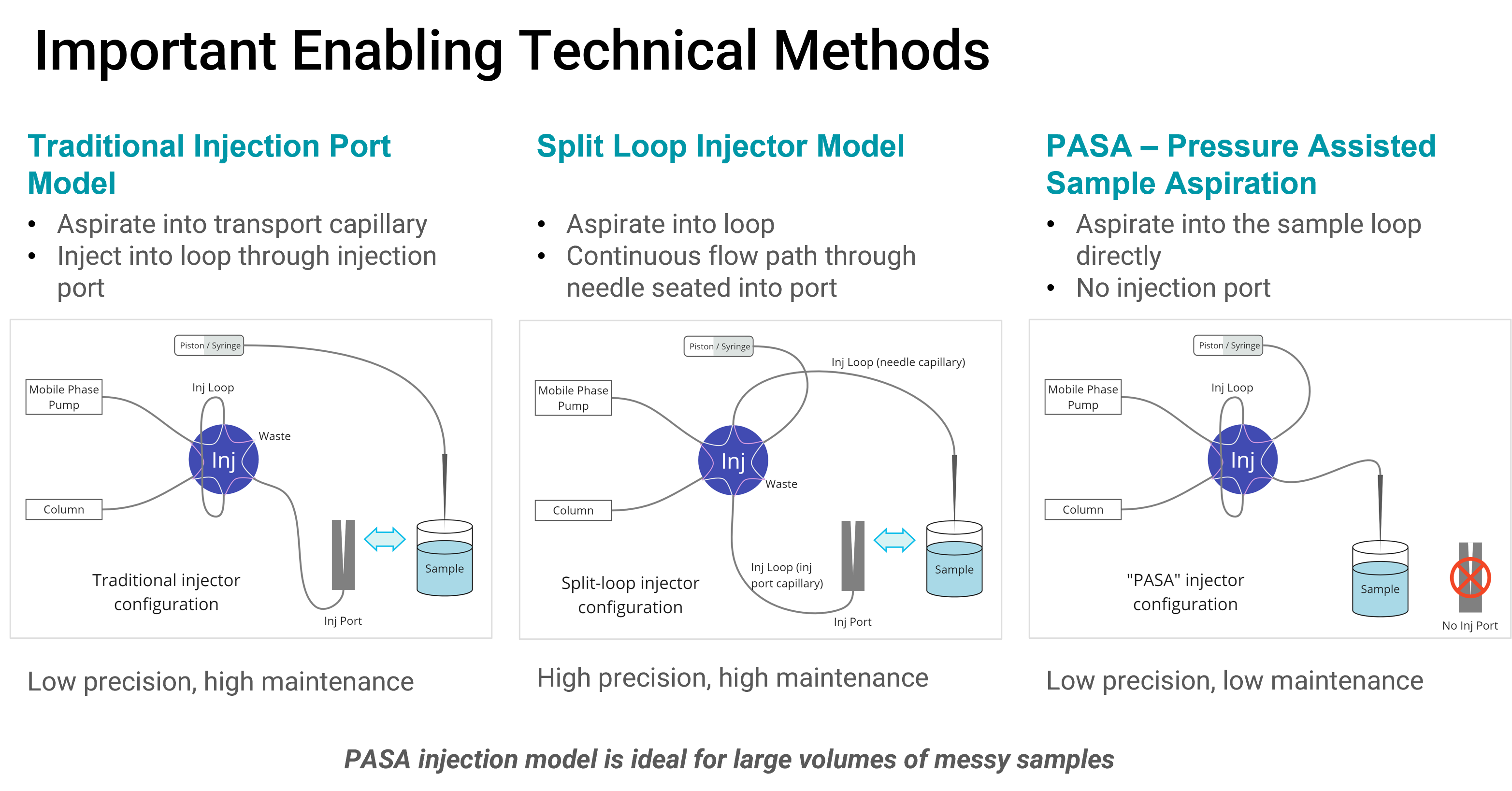
Important Enabling Technical Methods
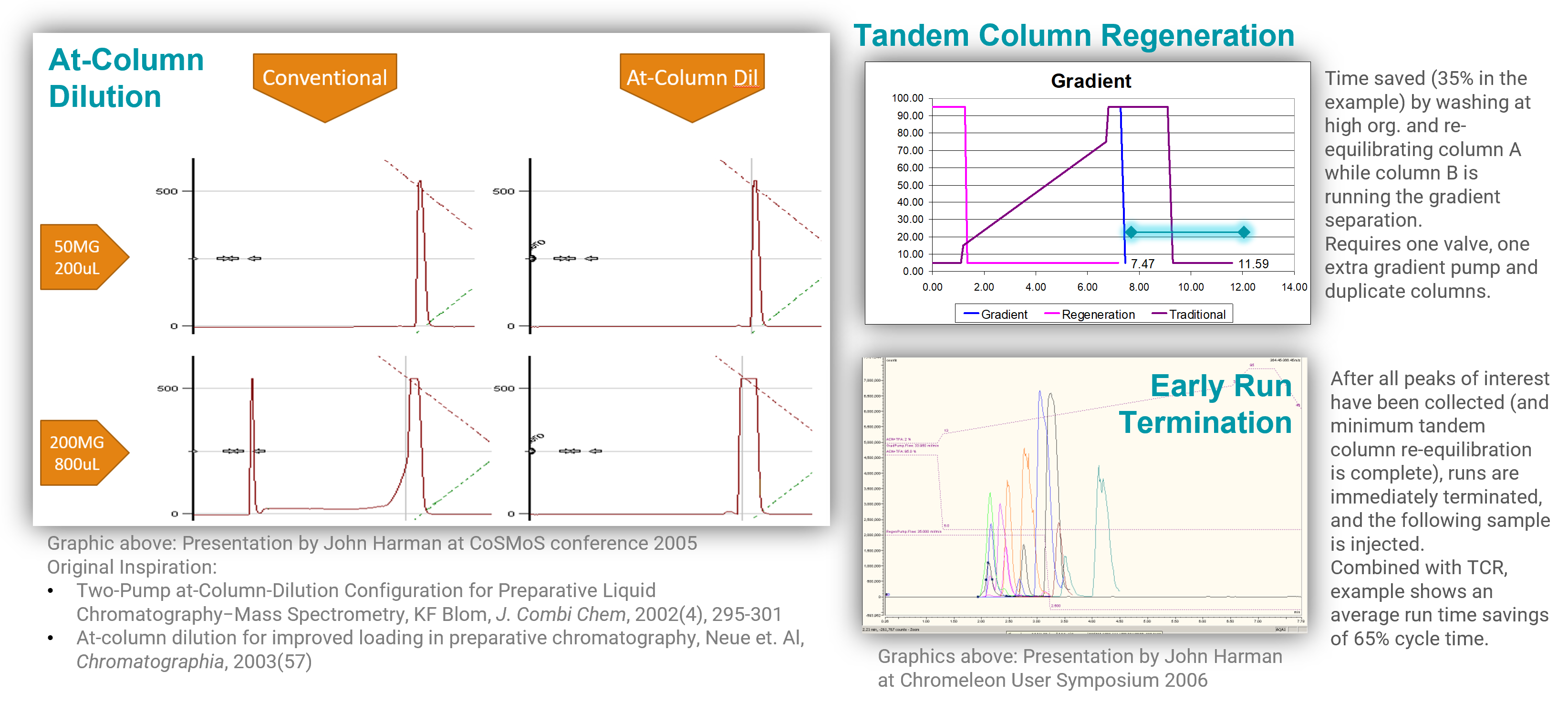
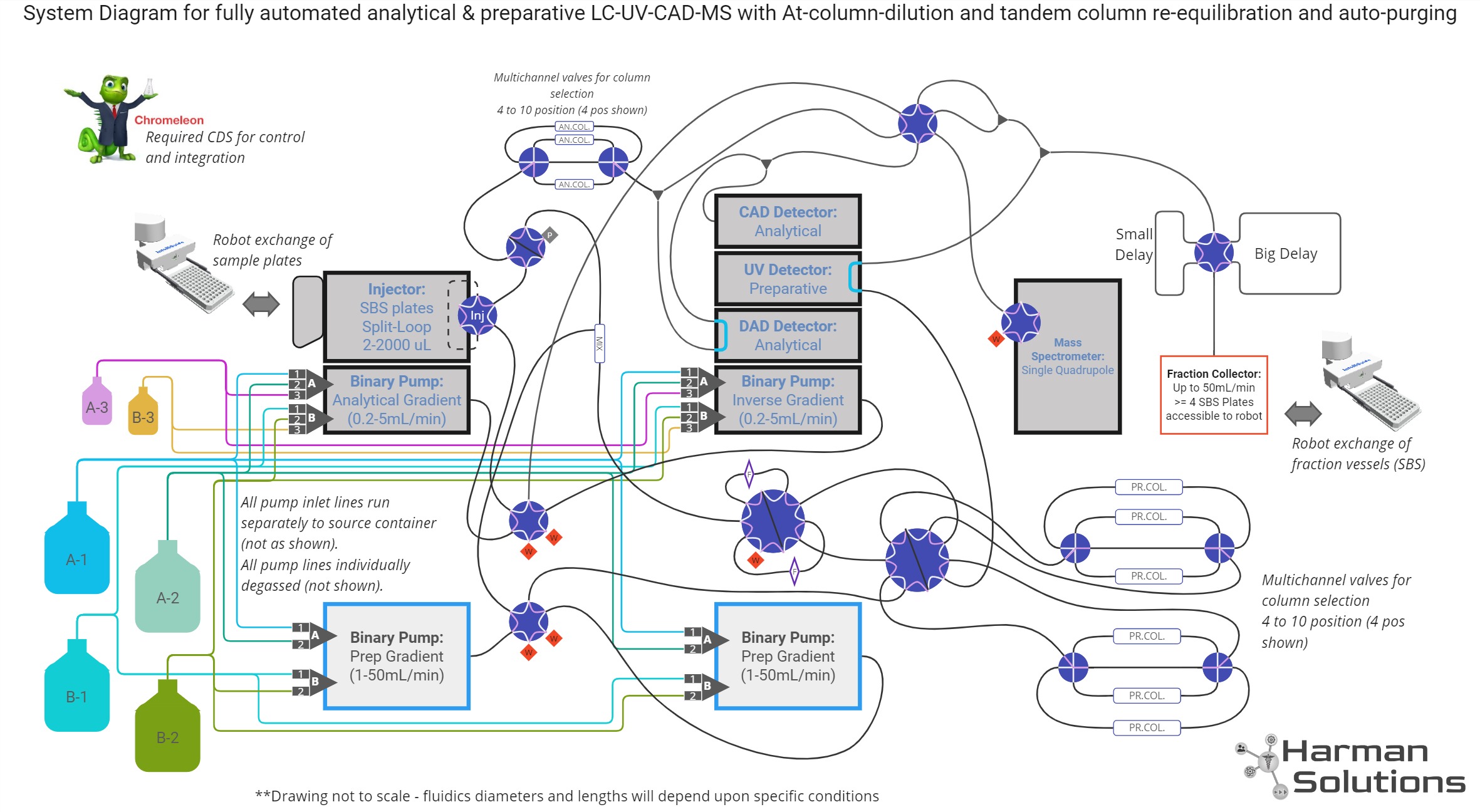
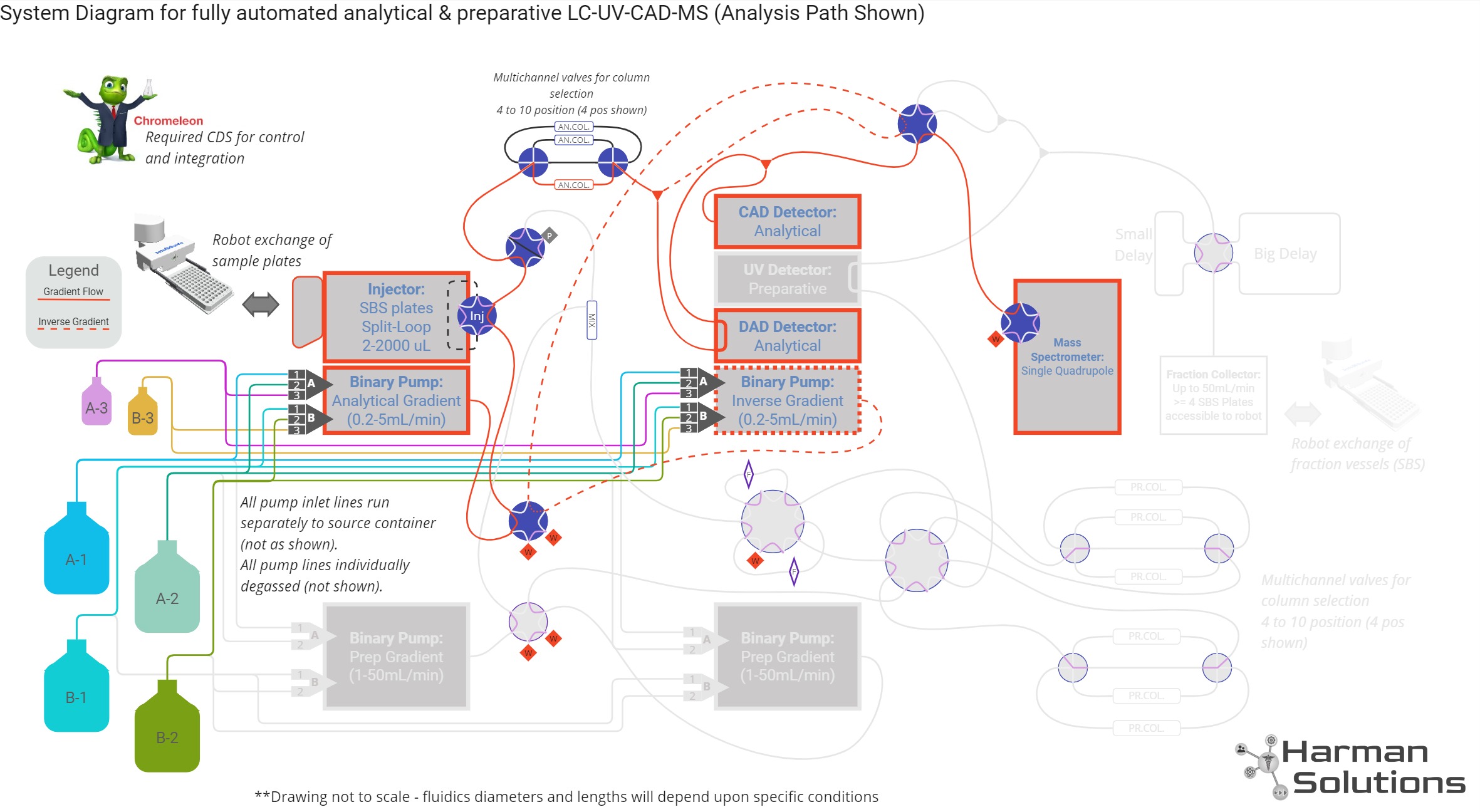
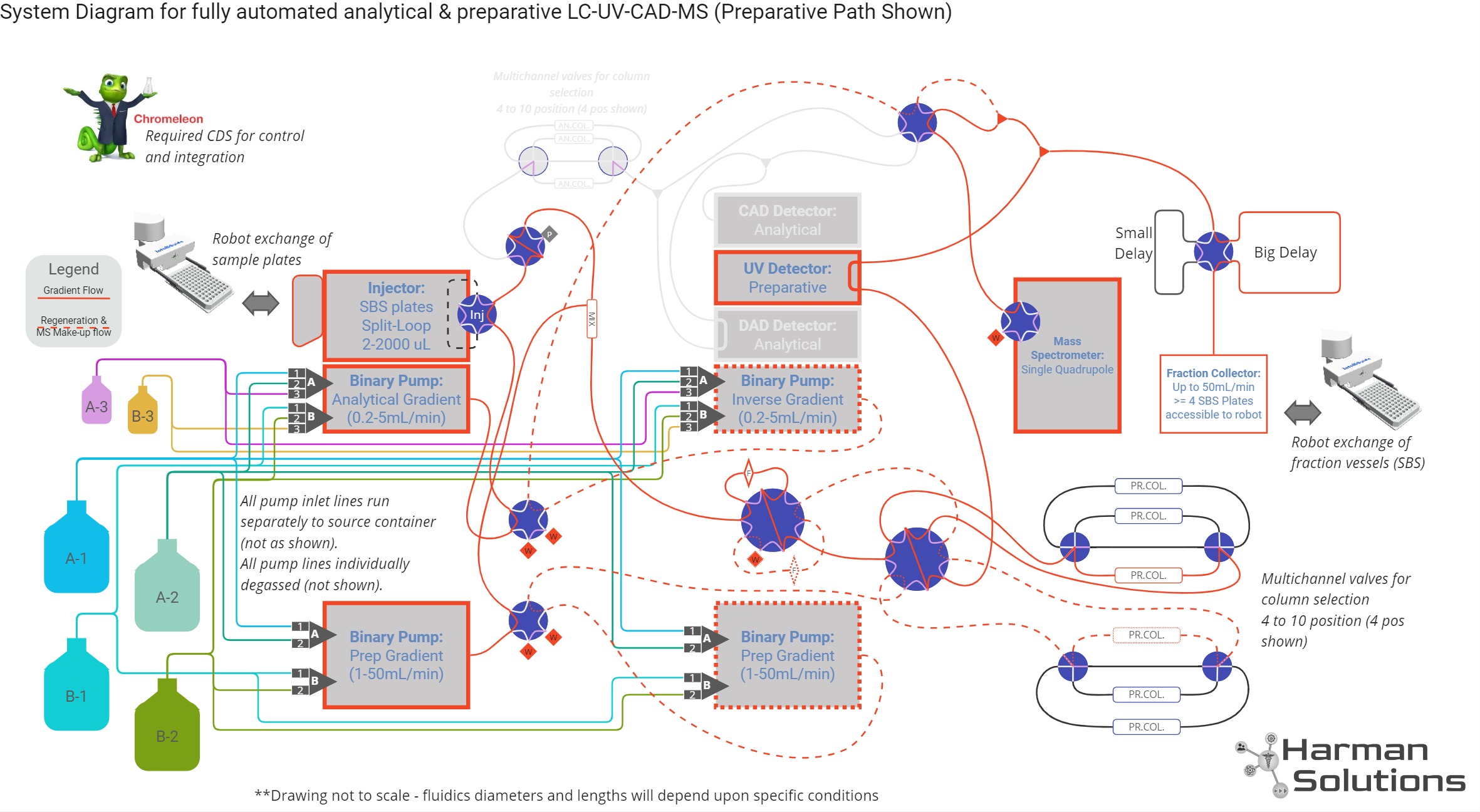
System Design
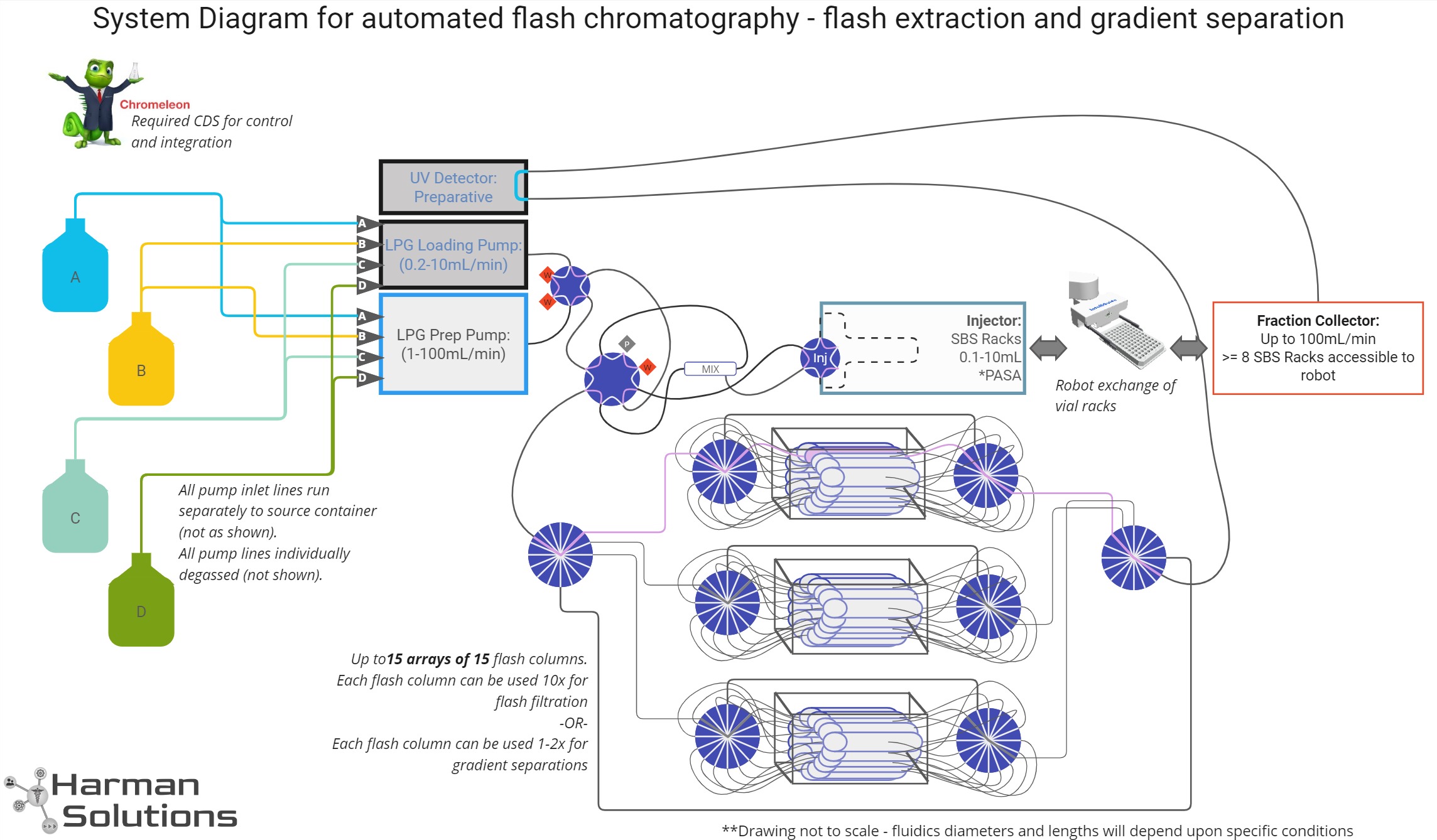
“Intermediate Scale” Purification
System concept is designed for sample scales of 0.1 - 5.0 g per purification
System Design Objectives
- Extract reaction products from crude reaction mixtures and solvents so they can be used in subsequent reaction steps
- Isolation (purification | extraction) of one or more specific molecules from a crude mixture with sufficient purity and yield for use in subsequent reaction steps
System Solution Design
Normal-phase serial flash chromatography system
A Novel Use of Technology You Use Every Day
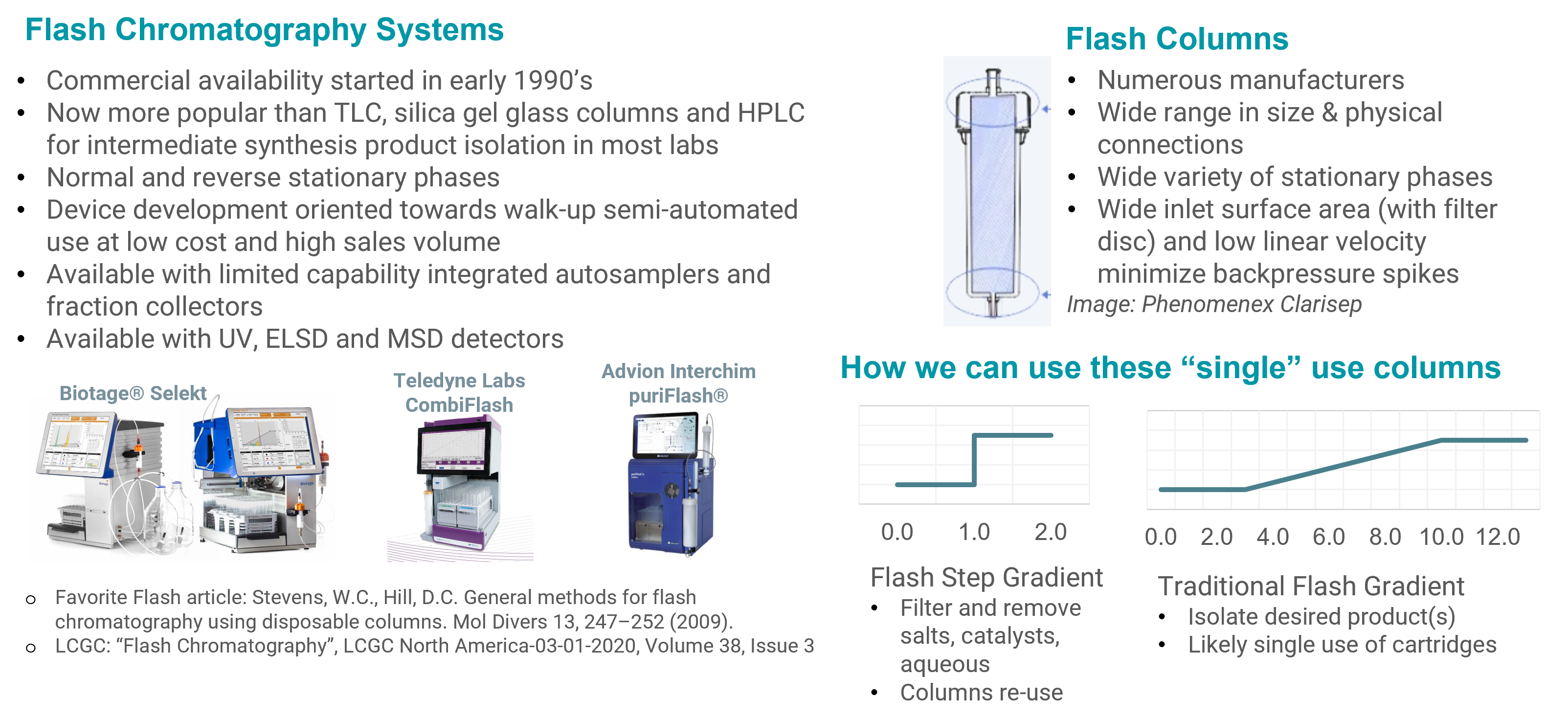
System Design
NOTE: The following design has never been built (that we know of). While it is simple in concept and uses common existing technology and methods in a novel way, someone will have to be the first…
Final QC Analysis
System Design Objectives
- Verify (not elucidate) the structure of target molecules
- Measure target molecule purity
- Measure quantity of target molecule
- Orthogonal separation conditions from purification / isolation
System Solution Design
Reverse phase UPLC-HRMS system with Charged Aerosol Detection
Important Technical & Tactical Details
Charged Aerosol Detection
- Website - Thermo Fisher Scientific HPLC-CAD learning Center
- References list (Thermo Fisher Scientific)
- Calibrate using different molecules with different hydrophobic index at multiple concentrations (weight conc - e.g. mg/ml) using a standard gradient
- Accurate quantitation requires an inverse gradient post-column to ensure that the molecules analyzed in the detector are measured under isocratic conditions
- A correction factor can be applied using a quadratic fit factoring in additional chemical properties (area of ongoing research)
- Absolute error ranges from 10-20%; however, this is far lower than weighing small amounts of material with assumed salt stoichiometry (typically closer to 30% error) and negligible for early stage primary assay error rates.
- Daily calibration runs are recommended, which can be fully automated and incorporated into Preparative LC recovery verification workflows of your fully automated DMTA platform
High Resolution Mass Spectrometry
- Exact mass and isotope patterns differentiate between all non-identical molecular formulae and most structural isomers
- When coupled with separation conditions orthogonal to the method of purification (e.g., high pH v. low pH), the precision of HRMS provides sufficient confidence in molecular identity verification to eliminate NMR as a primary sample workflow
- Requires occasional calibration, which may or may not be automated depending upon choice of MS instrument; however, daily verification of exact mass accuracy can be executed during frequent CAD calibration and Preparative LC recovery runs
System Design
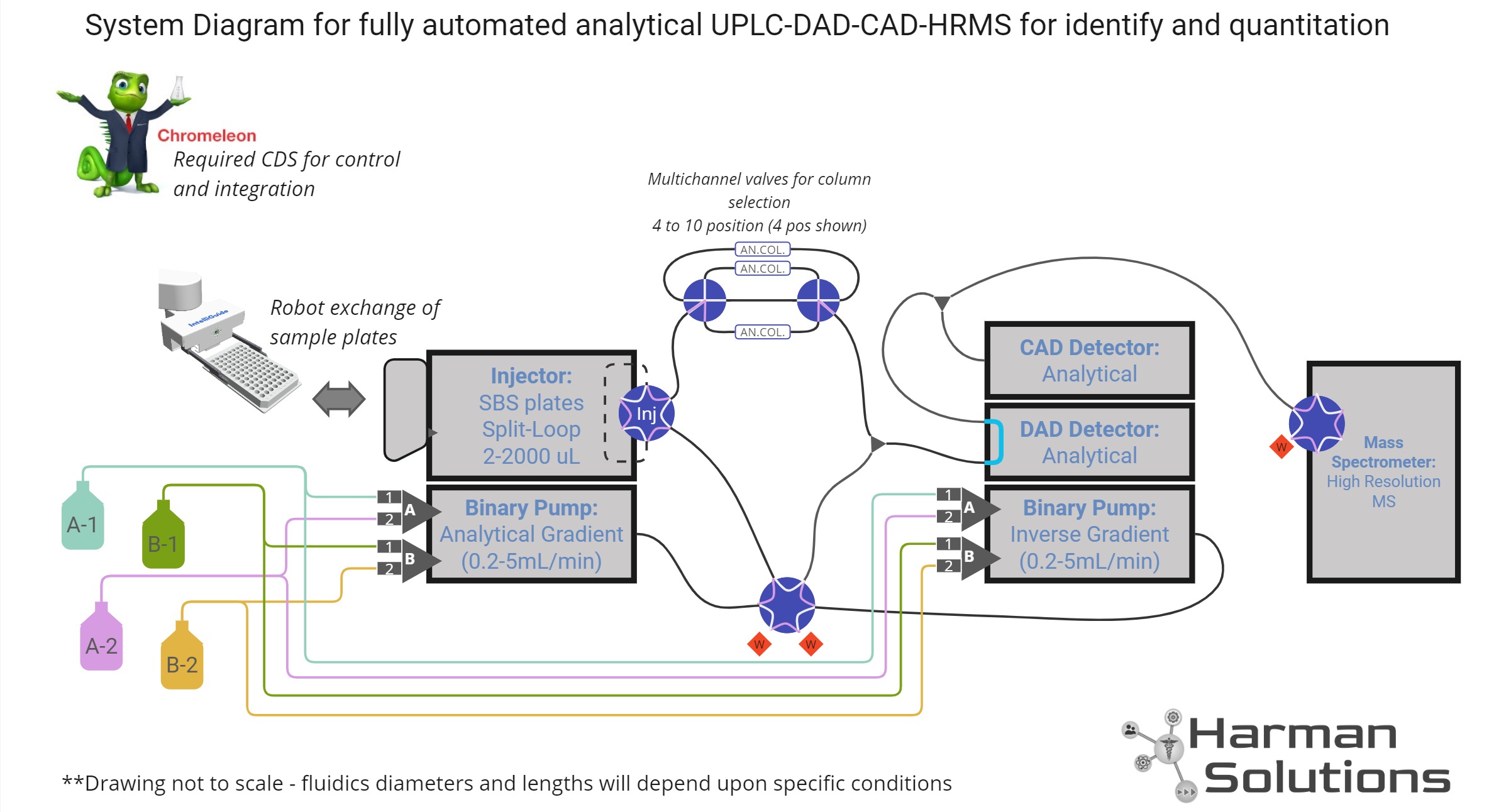
Concluding Comments
These opinions, tactics and system designs are intended to assist you in overcoming some of the significant challenges in fully automated chromatography. Many of the technical approaches, however, can be equally beneficial (robustness, efficiency) for the semi-automated and stand alone systems you use in your labs today.
Everything you have read here can be reproduced, modified, commercialized and otherwise used freely. It is not published in a proper journal because I do not believe in restricting access to important ideas our industry needs to accelerate.