SLAS2025 Automated DMTA Perspective
This year was my first year attending SLAS while NOT working for a specific company (exhibitor or attendee) and NOT focused on a specific set of new hardware to evaluate. Working across a wide variety of industry challenges as a consultant, this year my perspective was on the overall impact of new ideas or technology on solving the mediocre performance of drug discovery and development.
Context
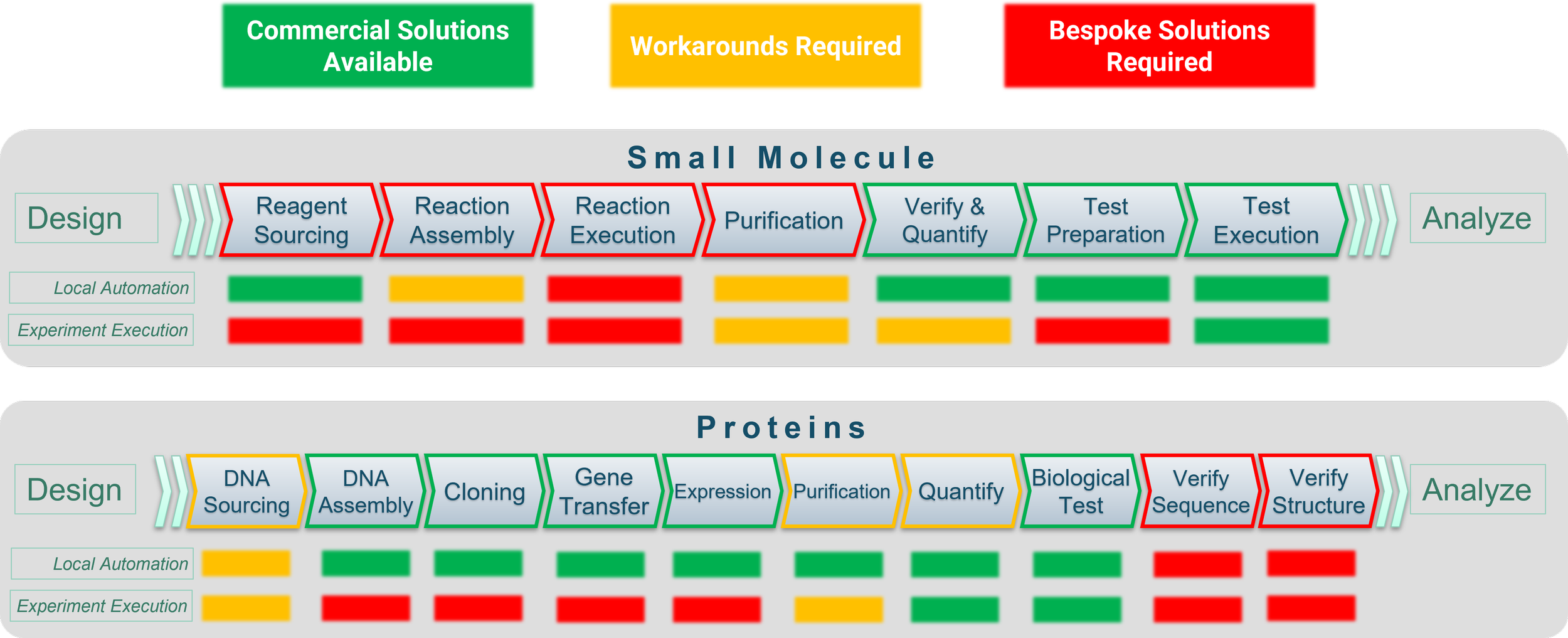
As a consultant specializing in the design and implementation of automated DMTA laboratories for both small and large molecules, my focus at SLAS 2025 was on evaluating new technologies that enhance drug discovery and development. I aimed to identify key advancements and remaining gaps in automating the Make + Test aspects of DMTA, specifically excluding in-silico design and analysis tools. This review is based on my scorecard of commercial solutions and best practices, highlighting areas which still require bespoke solutions for unsolved problems.
Reading these scorecards: Chevrons represent availability and feasibility of hardware. Local automation indicates the robustness of automation control software. The third and final row in each scorecard represents the availability of software for a research scientist (not automation engineer) to define and execute a scientific workflow and initiate execution without physical intervention.
Small Molecule DMTA
Nothing new at SLAS 2025 to help automate the process of finding, procuring, storing, and retrieving chemical reagents, but there were some other improvements.
Liquid Handling for Chemical Reagents
While most liquid handling is still in a race to the bottom on scale, which does not matter anymore BTW, there is one notable new tech which can significantly help with handling chemistry reagents. The Flow i8Ⓡ from FormulatrixⓇ is the first integratable, multi-channel liquid handler using positive displacement disposable tips with a volume range suitable for discovery scale chemistry applications. Why does this matter? Positive displacement and their liquid-class-less tech means you can handle any liquid without constraints about volatility, viscosity, or compatibility. Any reagent you wish is a neat liquid or that you can manage as a stock solution is solved - a major simplification.
Automated Solid Handling
Still NOT SOLVED. However; I’ll mention that a secret weapon I have been including in my designs for years is ramping up their presence. Sirius Automation an Addtronics Company displayed their small LibraryDoserⓇ device at SLAS. This isn’t the right device for a fully automated chemistry; however the offer numerous other robotic devices. Most important is a little tiny cap they make which fits on 1DR or 20mL vials. This magical cap contains an internal archimedes screw which, when used in their automation, can dispense well behaved solids into a vessel on a balance. When coupled with the right storage solution, thousands of solids can be retrieved and dispensed to support chemistry.
An Important “TIG”
Okay, what is a “TIG”? SLAS facilitated several “Topical Interest Groups” outlined here. One of these was for Automated Chemistry with Todd De Collo, Marc Feiglin, Derrick Miyao and Ruth Petersen. I did not attend in real time, but catching up with some of the panelists afterwards informed me that a primary objective is to standardize the labware (e.g. vials) in which reagents are stored and in which the reactions themselves are executed.
Mundane? Absolutely. Critically important? Absolutely - just look at the impact of the SBS Plate standard for the test side of DMTA. In case it isn’t obvious, this is my plea for industry to move quickly to adopt a version 1 of automated chemistry standards, garner support among industry, and continuously remind our vendors of what we need here.
Synthesis: No Reaction
There continues to be a void in technology for automating chemical reactions. While I found no tech examples on the floor, Ananda Herath from Novartis gave a talk on some work using HPLC pumps, an auto-sampler, and a heated segment of tubing. This was also coupled with LC to isolate the product. The “flow chemistry” debate has been around for decades. The advantage is linear scalability to generate product, which is incredibly appealing from an automation approach as one can use the same hardware for any scale of chemistry. The gap is how to manage the significant number of solid or sensitive reactants which are not easily introduced into these flow paths without human intervention.
In reality, executing chemical reactions is nothing more than moving reaction vessels of a few sizes (see the “TIG” above) into various heat, cool, agitation, pressure and light environments. This is an easy problem to solve mechanically if the vendors see a market demand. The demand is the problem. We chemists are consistently hung up on enabling every reaction instead of focusing on the top 10, top 20, even focusing on the top 75 would provide access to an incredible array of automated chemistry products, leaving only a small fraction to manual chemistry.
Automated Aqueous Evaporation - Critical Enabler
The Genevac EZ-2 4.0 Bionic Centrifugal Evaporator from ATS Scientific Products solves a very specific problem that has existed for over 25 years. This device enables evaporation of reverse phase chromatography fraction (required by 50-60% of all small molecules and capable of 99%) without human intervention. I also want to mention that the data output of this device enables ML driven evaporation configurations, further improving automated workflows.
Refining the use case here, if you are evaporating organic solvents use a nitrogen blow-down mechanism which is much cheaper. If you are thinking of using this device to evaporate DMSO - you can, but you shouldn’t - I always advise to avoid evaporating DMSO but rather avoiding the need by using a better process.
Automated Chromatography - New Examples of Existing Tech
There was a display in the NexusXp™ pavilion and some speaker content about examples of automated small molecule analysis and purification using chromatography. Acknowledging that the examples required tremendous effort, ingenuity, and risk tolerance by the researchers who implemented this automation. Each case involved many different software and hardware tools with significant constraints on the chromatography capabilities whichshould have off-the-shelf solutions available with some modest effort by system providers.
Somewhat concerning is that the hardware and software providers in this space continue to separate capabilities, which makes integration and total automation far more difficult. While analytical scale chromatography has 2 straight-forward easily integrated off-the-shelf solutions, purification requires 3 to 4 different hardware providers with conflicting control software which is either decades old or is actively being updated to target academic lab users (making it less capable of automated, industrial labs).
Testing Small Moelcules - Solved
The sample logistics, compound storage and in-vitro testing of molecules saw an important “step change” when acoustic liquid transfer technology became available. While a number of incremental innovations have occurred since, the downstream testing process for small molecules has remained the same. Bottom line…this is a solved problem. Yes, instrument companies can make incremental improvements and earn money for their investors and shareholders, but this slice of the DMTA cycle is already so fast relative to make that nothing which occurs here will have a net improvement on new patient treatments.
Automated DMTA for Proteins
An interesting thing happens when you shift into protein product molecules as your DMTA sample type. The make + test process is actually inverted primarily due to the following factors:
Constrain the molecular synthesis to a small number of chemical reactions (e.g. amino acid coupling)
Constrain the reactants (natural amino acids)
Add 3 orders of magnitude to molecular size
Introduce tertiary structure constraints
At the beginning of the make + test process, the supply chain challenges can now be feasibly solved by the nucleotide “fab shops” and the synthesis shifts into “managed” biological systems. The downstream operations become more complex due to the overall complexity of the molecular structures, which is where we find most of our challenges for full automation.
Verify Your Proteins?
Advances over the past 8 years have provided a number of tools to streamline the making of proteins and testing of proteins in therapeutically relevant assays. The typical workflow has naturally evolved to test first, verify later due in large to the lack of technology to confirm definitive protein structures. A number of non-integration friendly devices with narrowly scoped function exist, but we are only now seeing structure verification tools which are as broadly applicable to proteins as LCMS or NMR are for small molecules. So what are these technologies…well there are several but that’s going to be a different blog because nothing was featured, discussed or even hinted at in SLAS. Feels like a major scientific gap for the automation community.
Protein Purification Notables (not game changers)
I don’t believe throughput and capability constraints exist in fully automated purification of proteins as it does with small molecules; however, there are some notable technology improvements which are meaningful augmentation to existing techniques.
The BlueCatBio BlueⓇCombiX (not to take away from the device engineering) is so obvious it hurts. A system which can perform traditional centrifugation (not hi G forces) and inverted (wash) centrifugation using the same horizontal drum is very clever - and yet has been right in our faces since centrifugal washers arrived on scene (from BlueCatBio). For a fully automated system, this is good for a wide variety of applications including mag bead based protein extraction and routine liquid focusing and decanting in plates.
DPXⓇ Technologies has a few disposable liquid handler tips which cover basic filtration, nucleic acid extraction, solid phase extraction (e.g. functional protein retention) and even size exclusion (big/small separations). With some limitations related to liquid handler selection, a single source for multiple extraction steps within an automated platform can simplify your lab operations and accelerate your automated process development.
Conclusion
SLAS 2025 showcased modest progress in automating previously neglected areas of the DMTA loop. We're seeing important advancements that help close that loop, but there's still a key shift needed. We must move away from creating academic-focused instruments and instead prioritize the development of robust, industrial-grade scientific tools and software. This shift is crucial to help us work faster, more cost-effectively, and with higher success rates.
For true automated DMTA, we need more than just automating complex processes. We need to empower scientists to design new experiments, review the autonomously generated results, and iterate on their hypotheses seamlessly. While current tools handle repetitive development workflows well, early-stage research still lacks the automation needed to truly unleash scientific innovation. It's time for the automation community to address this gap and provide solutions that meet the needs of scientific innovators.
John Harman, Harman Solutions LLC, 2025
DISCLAIMER : All content is based upon SLAS2025 show observations and the opinions of John Harman. If inaccuracies exist, they originate from inaccurate show exhibit, documentation, or information provided by vendor representatives.